Early Peanut Oral Immunotherapy (EPOIT) can decrease the severity of peanut allergy in young children. Say what?!
Let’s discuss early peanut oral immunotherapy (EPOIT).
“So let me get this straight: she’s allergic to peanut. But if we slowly start giving her tiny amounts of peanut, her allergy can decrease?“ Baby Sweetpea’s mom asked her allergist.
Her allergist replied, “That pretty much sums it up.”
What is OIT?
OIT—oral immunotherapy—is a treatment. It is not a cure. It is a treatment for food allergy.
This treatment involves slowly exposing a person’s immune system to the person’s allergen. That’s the “immunotherapy” part. In food allergy, the most common route of immunotherapy is by mouth. That’s the “oral” part. In peanut OIT, the peanut-allergic patient ingests peanut.
Check out my earlier infoblog post on OIT.
What is early peanut oral immunotherapy (EPOIT)?
Early peanut oral immunotherapy (EPOIT) is a form of immunotherapy for young children.
Children ages 4 months through 3 years typically define “early” in early OIT. These patients have peanut allergy. This means they have an allergic reaction to peanut. Sometimes, however, babies who have not yet ingested a peanut-containing food but have elevated allergy tests or other risk factors may be candidates for early peanut oral immunotherapy.
Depending on the allergist performing EPOIT, the form of peanut used as treatment can vary. Of course, nuts are choking hazards in this age group. Because of this hazard, EPOIT protocols use peanut powders, peanut flours, and peanut butters diluted with water. Just as with OIT in older age groups, patients undergoing EPOIT ingest daily doses of the specified peanut product. The starting dose of therapy is super low, often microgram doses! Patients go into the allergist’s office every 1-2 weeks for up-dosing of their treatment. They do this until they reach their maintenance dose. Maintenance dosing varies per protocol. Allergists typically continue maintenance dosing for at least one year before repeating allergy testing. Patients undergo EPOIT under the strict supervision of food allergists.
Early peanut oral immunotherapy (EPOIT) is what I often refer to as LEAP 2.0. Let’s revisit LEAP…
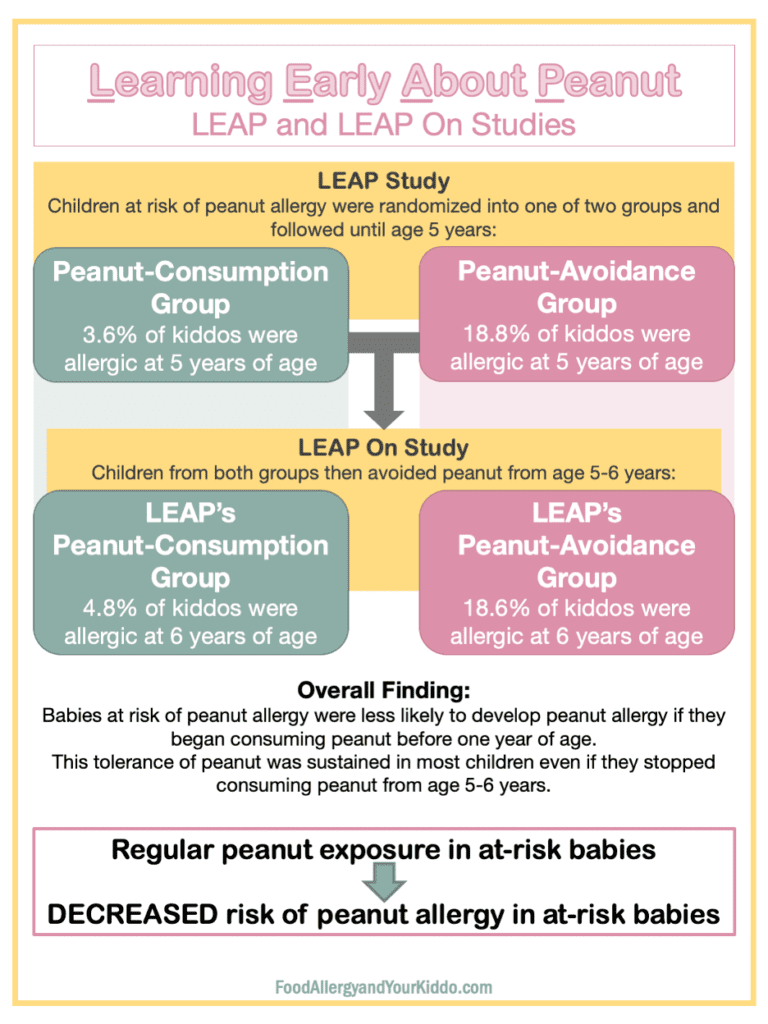
LEAP is the study “Learning Early About Peanut.” In this study, babies (4-11 months of age) who were at risk of peanut allergy were stratified into one of two groups: peanut-consumption group or peanut-avoidance group.
- “At risk” of peanut allergy means the baby had 1) severe eczema, or 2) egg allergy.
- All babies had skin prick testing (SPT). Read more about allergy testing here.
- Positive (+) SPT = 1-4 mm wheal
- Negative (-) SPT = 0 mm wheal
- SPT >4mm = the study excluded these babies.
- The study defined “peanut consumption” as 6 grams of peanut protein per week. Bamba served as a main form of peanut protein.
The primary outcome of LEAP was whether or not babies were allergic to peanut at 5 years of age. Ingestion challenge determined the presence of peanut allergy. An ingestion challenge is a procedure in which a patient consumes the allergen in question and is monitored for symptoms. More about that here.
LEAP found that at-risk children who consumed peanut were significantly less likely to have peanut allergy by their 5th birthday when compared to at-risk children who had been avoiding peanut.
The LEAP On Study immediately followed the LEAP Study.
LEAP On tested the sustainability of this tolerance (read about allergy vs. tolerance here). In LEAP On, babies from both the peanut-consumption and peanut-avoidance groups were advised to avoid peanut for one year. They were then tested for peanut allergy at six years of age. Peanut ingestion challenge determined allergy status.
LEAP On found that children who had consumed peanut until five years of age were still significantly less likely to have peanut allergy after a year of peanut avoidance compared to at-risk children who had been avoiding peanut since they were babies.
New(ish) Recommendations for Introduction of Peanut Products to Babies
So now maybe you are wondering…
“Even an at-risk baby with positive skin testing can be tolerant of peanut if the baby begins consuming peanut at a young age. But in LEAP, babies with SPT >4mm were excluded. So should babies who have SPT >4mm avoid peanut?”
Not necessarily.
After LEAP and LEAP On, food allergy management recommendations changed. In 2017, the National Institute of Allergy and Infectious Disease (NIAID) published new recommendations. These new recommendations are summarized in the table “Summary of the NIAID Peanut Introduction Recommendations.” In short, if a baby is at risk of peanut allergy s/he should be further evaluated with allergy testing. If s/he is not at risk, then age-appropriate peanut products can be introduced as early as 6 months of age. This is in keeping with the WHO and AAP recommendation of exclusive breastfeeding for the first 6 months of life.
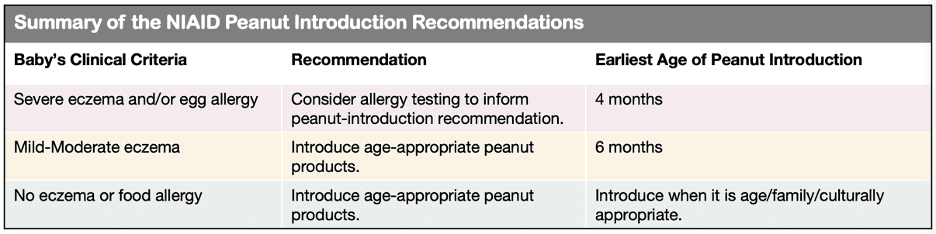
Age of introduction is based on risk factors for peanut allergy.
“But what if my baby has milk allergy? Or what if her brother has peanut allergy? Are those risk factors?” Not according to these newest guidelines. That is why it’s important to see an allergist—so that your child’s evaluation can be personalized to her clinical presentation.
Peanut Allergy Prevention Recommendations In Action
At-risk babies are evaluated for likelihood of peanut allergy through skin and/or blood allergy testing. Which testing your doctor performs typically depends on whether or not the doctor is an allergist. Most pediatricians and family medicine doctors do not have access to skin testing, so they may order a blood test . If the blood allergy test is negative, then allergy is unlikely. The doctor then may advise introduction of an age-appropriate peanut product.
In my experience, many non-allergy docs prefer referring at-risk patients to the allergist for further evaluation. While the allergist may perform blood testing, s/he is also likely to perform skin prick testing. Based on the NIAID recommendations, skin prick testing provides guidance on whether peanut should be introduced and, if so, how. Check out the table labeled “Summary of NIAID’s Peanut Introduction Recommendation Based on Skin Testing.”
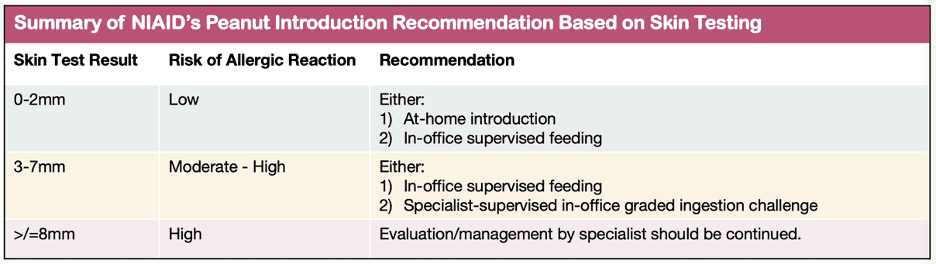
Method of introduction in babies at risk of peanut allergy is based on history and test results.
Application of Recommendations to Prevent Peanut Allergy
All that being said, many food allergists perform in-clinic peanut challenges despite a positive blood test and/or a large skin prick test. This is because neither skin testing nor blood testing are not 100% accurate for diagnosing food allergy (more on that here). Ingestion challenge is the gold standard to make the diagnosis of food allergy. If a child has a negative peanut challenge, then the allergist likely will recommend continued ingestion of peanut to sustain that tolerance. If a baby as a positive peanut challenge, avoidance is often recommended…
But not always. Not anymore! This is when early peanut oral immunotherapy can be helpful.
Please note: early peanut oral immunotherapy is not an FDA-approved treatment for peanut allergy.
There is no FDA-approved treatment for peanut allergy in this age group. The only FDA-approved treatment for peanut allergy is Palforzia. The FDA approved the initiation of treatment in patients ages 4-17 years old. Patients older than 17 years can continue treatment. This means that children younger than 4 years old and adults do not meet criteria for Palforzia.
But do some allergists do OIT other than Palforzia, using food or food powders/flours? Yes.
Safety and Efficacy of Early Peanut Oral Immunotherapy
Do some allergists do early peanut OIT? Yes. But is it safe? Let’s go through a few journal articles to answer that question.
Article #1: Vickery et al studied early peanut OIT in a research setting.
This is one of my favorite papers, the title of which is “Early Oral Immunotherapy in Peanut-Allergic Preschool Children Is Safe and Highly Effective.” In this study, preschool-aged children with peanut allergy received either low or high dose peanut oral immunotherapy. Not only were both doses quite successful in inducing tolerance to peanut, but the low dose of peanut protein seemed equally as effective in inducing tolerance but with a better side effect profile than the high dose.
Most of the children in the study achieved sustained unresponsiveness. Sustained unresponsiveness occurs when somebody who previously had a food allergy undergoes OIT and is then able to ingest the food without having an allergic response to it. This study followed these children after five years, and most of them continued to ingest peanut without having reactions. One of my favorite parts of this story is that the families reported an improved quality of life.
Article #2: Soller et al studied early peanut OIT in a community setting.
Soller’s team performed EPOIT—which they referred to as preschool P-OIT—and confirmed the safety findings of Vickery’s research but in a community setting. Regarding feasibility, this real-world approach to EPOIT used real food, specifically peanut butter powder and Bamba. Vickery’s study, used encapsulated peanut protein.
Note the Safety…
There were children from both cohorts who did have allergic reactions. Rarely, these reactions were severe. This underscores the importance of the strict supervision and guidance of a food allergist for patients undergoing oral immunotherapy.
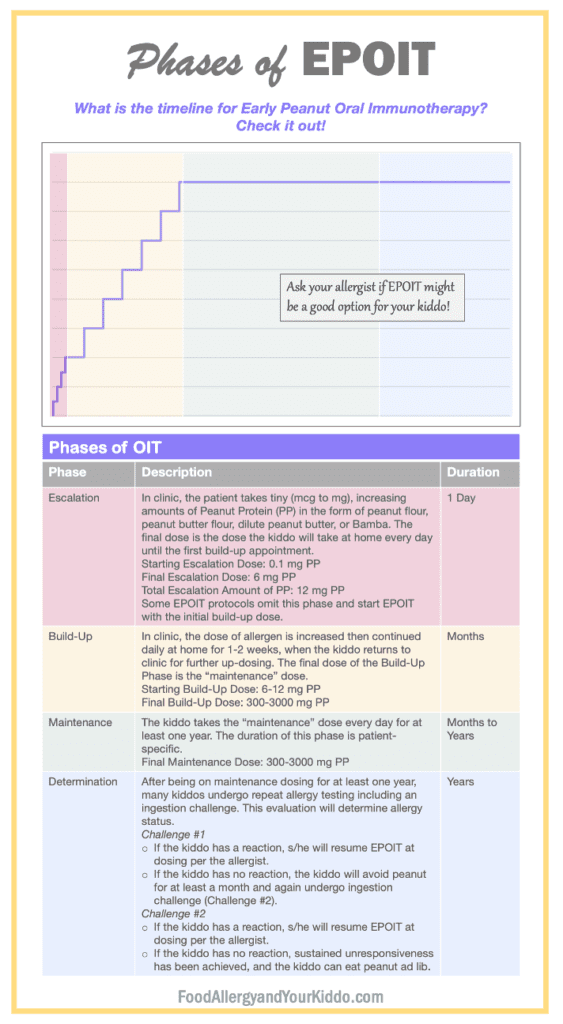
Each phase of EPOIT is performed under strict guidance of the kiddo’s allergist.
Note: one peanut contains approximately 250-300 mg Peanut Protein (PP).
Note the Controversy…
Most allergists agree that oral immunotherapy is effective. That being said, allergists don’t always agree on how OIT is administered. Many allergists feel that OIT should be administered only when using an FDA approved product, such as Palforzia. Otherwise, OIT should only be used in a research setting. Others feel that using foods from the supermarket is equally effective and safe. Ask your allergist for his/her thoughts.
Is Early Peanut Oral Immunotherapy (EPOIT) right for your child?
Ask your allergist.
EPOIT may or may not be the best approach to your child’s peanut allergy at this time. Regardless, you are now more informed on this therapy.
So much information about food early peanut oral immunotherapy (EPOIT)!
We’ve covered a lot in this infoblog post! I imagine you must have some questions. Send them my way!
Have your peanut oral immunotherapy and other food allergy questions answered on the podcast! Submit your question HERE!
Additional Notes
I have talked about a non-profit…
The non-profit is The Teal Schoolhouse, whose primary program is Code Ana. Code Ana equips schools for medical emergencies like anaphylaxis. Our primary program is the Code Ana School Program, which is a comprehensive approach to school-focused medical preparedness. This program guides schools through the process of creating a medical emergency response plan. This is one of the most important components of a school’s food allergy policy!
A medical emergency response plan is important for all kiddos and for adults at any school! Our primary goal is to share the School Program, and Code Ana’s Online Epinephrine Training Program helps support that goal. Through this program, you will educate yourself while you support this important mission! (BTW although Pam and I serve in leadership roles of Code Ana and The Teal Schoolhouse, our time/effort/work is completely voluntary). Does your kiddo’s school have Code Ana?